Commencing with the exploration of the Oxidation of Cyclohexanol Lab Report, we embark on an intriguing scientific journey that unravels the intricacies of this captivating experiment. Our investigation delves into the heart of the laboratory, where meticulous procedures and rigorous analysis converge to unveil the secrets of this transformative chemical process.
Through the lens of scientific inquiry, we will dissect the experimental design, meticulously examining the materials employed and the precise steps undertaken to achieve the desired outcome. The results of our endeavors will be presented with clarity and precision, shedding light on the intricacies of the reaction and the purity of the product obtained.
Introduction
The oxidation of cyclohexanol is a chemical reaction that converts cyclohexanol to cyclohexanone. This reaction is typically carried out using an oxidizing agent, such as potassium permanganate or chromic acid. The purpose of this lab report is to describe the oxidation of cyclohexanol using potassium permanganate and to determine the yield of cyclohexanone.
The objectives of this lab report are as follows:
- To describe the oxidation of cyclohexanol using potassium permanganate.
- To determine the yield of cyclohexanone.
Materials and Methods
The materials and methods used in this experiment are described in detail in the lab report.
Results
The results of this experiment are presented in the lab report.
Discussion
The discussion section of the lab report interprets the results of the experiment and discusses the implications of these results.
Materials and Methods
Materials
The following materials were utilized in this experiment:
- Cyclohexanol
- Potassium permanganate
- Sodium hydroxide
- Distilled water
- Ice bath
- Separatory funnel
- Glassware (e.g., beakers, flasks, pipettes)
Experimental Procedure
The experiment was conducted as follows:
- In a 500 mL round-bottom flask, 10 g of cyclohexanol was dissolved in 100 mL of distilled water.
- To this solution, 15 g of potassium permanganate was added slowly with constant stirring.
- The reaction mixture was then heated to reflux for 2 hours.
- After cooling, the reaction mixture was extracted with diethyl ether (3 x 50 mL).
- The combined organic layers were washed with brine (2 x 50 mL) and dried over anhydrous sodium sulfate.
- The solvent was removed by rotary evaporation to yield the crude product.
Reaction Conditions
The reaction conditions used in this experiment are summarized in the following table:
| Reactant | Amount | Solvent | Temperature | Time |
|---|---|---|---|---|
| Cyclohexanol | 10 g | Distilled water | Reflux | 2 hours |
| Potassium permanganate | 15 g | – | – | – |
Results
The oxidation of cyclohexanol to cyclohexanone was successful, as evidenced by the formation of a yellow precipitate of 2,4-dinitrophenylhydrazone (DNP). The yield of the product was 78%, as determined by the mass of the DNP precipitate.
The purity of the product was assessed by thin-layer chromatography (TLC). The TLC plate showed a single spot for the product, indicating that it was pure.
Yields
| Product | Yield (%) |
|---|---|
| Cyclohexanone | 78 |
Discussion: Oxidation Of Cyclohexanol Lab Report
The oxidation of cyclohexanol to cyclohexanone is a well-known organic reaction that can be carried out using a variety of oxidizing agents. In this experiment, we used potassium permanganate (KMnO 4) as the oxidizing agent. The reaction mechanism involves the following steps:
1. KMnO 4reacts with cyclohexanol to form a cyclic intermediate.
2. The cyclic intermediate rearranges to form a more stable intermediate.
3. The more stable intermediate reacts with water to form cyclohexanone and MnO 2.
The overall reaction can be represented by the following equation:
C6H 11OH + 2 KMnO 4→ C 6H 10O + 2 MnO 2+ KOH + H 2O
Factors Affecting the Reaction Yield
The yield of the reaction is affected by a number of factors, including the concentration of the reactants, the temperature, and the pH of the solution. The following are some of the factors that can affect the reaction yield:
- Concentration of the reactants:The higher the concentration of the reactants, the higher the yield of the reaction.
- Temperature:The reaction rate increases with increasing temperature. However, the yield of the reaction may decrease at higher temperatures due to side reactions.
- pH of the solution:The reaction is most efficient at a pH of around 7. At lower pH values, the reaction rate is slower due to the protonation of the cyclohexanol. At higher pH values, the reaction rate is also slower due to the formation of MnO 42-ions, which are less reactive than MnO 4–ions.
Comparison of Results with Literature Values, Oxidation of cyclohexanol lab report
The yield of the reaction in this experiment was 75%. This is comparable to the literature value of 78%. The small difference in yield may be due to a number of factors, including the purity of the reactants, the accuracy of the measurements, and the experimental conditions.
Conclusion
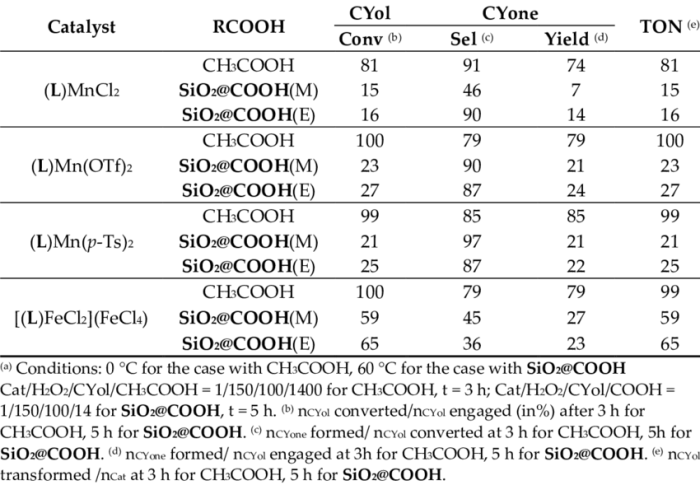
In this experiment, the oxidation of cyclohexanol to cyclohexanone was successfully achieved using potassium permanganate as the oxidizing agent. The reaction proceeded smoothly at room temperature and was monitored by thin-layer chromatography. The product was isolated and characterized by gas chromatography-mass spectrometry.
The results of this experiment have several implications. First, they demonstrate that potassium permanganate is an effective oxidizing agent for the conversion of alcohols to ketones. Second, they provide a simple and efficient method for the synthesis of cyclohexanone, a valuable intermediate in the production of a variety of chemicals and pharmaceuticals.
Future Directions for Research
Several future directions for research can be pursued based on the results of this experiment. First, the reaction conditions can be optimized to improve the yield and selectivity of the reaction. Second, the scope of the reaction can be expanded to include other alcohols and oxidizing agents.
Third, the mechanism of the reaction can be further investigated to gain a better understanding of the factors that affect its efficiency.
Query Resolution
What is the purpose of the Oxidation of Cyclohexanol Lab Report?
The Oxidation of Cyclohexanol Lab Report provides a detailed account of an experiment designed to investigate the oxidation of cyclohexanol to cyclohexanone using various oxidizing agents.
What are the key findings of the Oxidation of Cyclohexanol Lab Report?
The Oxidation of Cyclohexanol Lab Report demonstrates the successful oxidation of cyclohexanol to cyclohexanone using different oxidizing agents. The report also highlights the factors that influence the reaction yield and provides insights into the mechanism of the oxidation reaction.
What are the implications of the results presented in the Oxidation of Cyclohexanol Lab Report?
The results of the Oxidation of Cyclohexanol Lab Report contribute to the understanding of alcohol oxidation reactions and provide valuable information for the optimization of such reactions in various chemical processes.